Published via Research News on July 12, 2023
There is an upcoming change to the process* of requesting new Research Monitor accounts for EpicCare Link that goes into effect on Tuesday, August 1, 2023.
Research Monitors will be required to fill out and sign a DocuSign user form before their EpicCare Link account will be created. Research Monitors can access this user form here.
Research Monitors can also access the DocuSign user form directly by accessing MyEpicCareLink.org and clicking the hyperlink as referenced in the photo below.
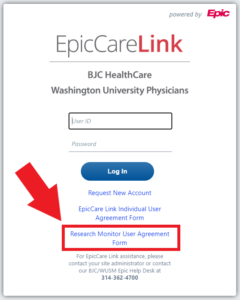
Rationale for requiring this DocuSign user form:
These are the new requirements for EpicCare Link as mandated by BJC Compliance, the WashU Privacy Department, and both Legal Departments. The completed form is required for EpicCare Link Managed Access and there are no exceptions. This process allows us to maintain the same level of oversight and accountability for research monitors that we require for BJC & WashU employees accessing Epic. User data is integrated with FairWarning and allows us to monitor all access to any patient information as required by law.
*This new DocuSign form must be completed in addition to the existing processes of the Research Monitor completing HIPAA training and the BJC/WU Study Team submitting the EpicCare Link new account request. Below are all of the steps involved in the process, with the new DocuSign user form step in bold. For more detailed information, please navigate to the Research Learning Home Dashboard in Epic and open the “HIM, Printing, and Study Monitors” heading in the Research Tip Sheets section.
- Research Monitor requests Guest WUSTL key
- N/A for non-WashU sites
- WashU Study Team approves Guest WUSTL key
- N/A for non-WashU sites
- Research Monitor completes “HIPAA Training for Research Monitors” in SABA
- For non-WashU sites, BJC Study Team sends HIPAA training certificate to Epic1 Research Team
- Research Monitor fills out and signs DocuSign user form
- BJC/WashU Study Team submits request for EpicCare Link account creation at MyEpicCareLink.org
- Epic1 Research Team confirms HIPAA training and DocuSign user form completion and creates EpicCare Link account
- Epic1 Research Team sends EpicCare Link account information to BJC/WashU Study Team via Epic In Basket
- BJC/WashU Study Team sends EpicCare Link account information to Research Monitor
- BJC/WashU Study Team releases patients to the patient group for the appropriate dates
Questions?
Contact epic1research@wustl.edu.