Proposal development requires a variety of individuals and offices across the university to provide key components for your research project. Here you can find the resources and offices needed to assist in the development of a proposal.
Proposal Development Resources
1: Planning Phase
The planning phase focuses on gaining knowledge, data, and partners to prepare for a future proposal or project. This may include competitive analysis of your concept/idea, workshops, generating white papers, finding collaborators, identifying mentors, contacting program officials, generating preliminary data, and talking with your Department Chair or Dean. Resources available to faculty during this phase include:
Developing Concepts and Ideas
Concept Development Resources and Services are offered through the Research Development Office, Medical Corporate and Foundation Relations, and Danforth Campus Foundations Relations or Corporate Engagement Offices. Faculty may also present concept ideas to peers, mentors, or program officials. Several federal agencies invite concept or white paper submissions on a rolling basis.
Understanding Federal Agencies and Research Landscape
Agency Information Resources including agency information webinars and Q&A panels, awards databases, strategic reports, and links to finding program officials. The Research Development Office offers competitive intelligence analysis and strategic planning for emerging teams.
Finding WashU or Regional Collaborators
The Research Development Office assists faculty in identifying collaborators across schools and disciplines, facilitates new team development, and hosts topic specific Research Networking Groups initiated by faculty.
Research Education and Information hosts an annual faculty forum with opportunities to foster collaborative relationships.
‘Find Collaborator Tools’ are available through WashU Profiles and SciVal.
Generate Preliminary Data
Internal Seed Funding opportunities provide funds to support research activities that may lead to extramural funding.
There are a variety of federal funding programs offering early career opportunities, as well as private sponsors found in Foundation Relation’s Private Funding Sources catalog.
Federal Funding & Cost Sharing
If you choose to pursue federal funding for your research project, be sure to check your eligibility to apply as a PI.
- School of Medicine
- All other disciplines should check with their School for current policies on PI eligibility.
2: Find Funding
The university has multiple resources to find funding from a variety of sponsor types, and several offices to support you in finding funding opportunities.
Submit a Concept Paper or White Paper
Some sponsors offer or require the option to submit concepts prior to the formal proposal submission.
- NSF Program Suitability & Proposal Concept Tool (ProSPCT): for prospective PIs to contact the NSF to determine suitability of a project idea prior to submission of a proposal.
- NSF Broad Agency Announcement Management (BAAM): a document management system for submission of letters of intent, preliminary proposals, and full proposals in response to a NSF BAAs.
Contact a Program Officer
“Engaging with Federal Program Officials” – Recording and Slides: Training provided at the 2021 Researcher Forum (Box links require WUSTL Key).
NIH: Program Officials Are Here to Help
NSF 101: 5 tips on how to work with an NSF program officer
Once your grant proposal has been submitted for review, it is not appropriate to contact individuals involved in the review process. The Peer Review Confidentiality Requirements announcement from the Vice Chancellor for Research details important information on confidentiality during the review process.
Review Funding Trends
Federal Agency Information: This resource connects you with current funding priorities from a variety of federal agencies, as well as recordings from recent federal agency presentations at WashU, hosted by the Research Development Office.
WashU Research Analytics (WUSTL Key required): A series of interactive dashboards useful for analyzing various aspects of extramural funding.
Account Registration for Submissions
WashU’s Institutional Data Quick Reference Sheet: Includes important information, like WashU’s UEI number, required for some system registrations.
FedConnect: lists opportunities for federal contracts, grants, and other types of funding.
eRA Commons: A system managed by NIH that allows applicants, recipients and federal staff to securely share, manage and process grant-related information. This application is submitted to your Department Administrator.
Application Submission System & Interface for Submission Tracking (ASSIST): is used to prepare and submit applications electronically to NIH and other Public Health Service agencies.
SciENcv: This tool allows researchers to track and share their biosketch and current and pending support. Biosketch samples can be found in the WUSTL Grants Library>Standard Grant Language Templates in Box.
3: Write & Compile Proposal
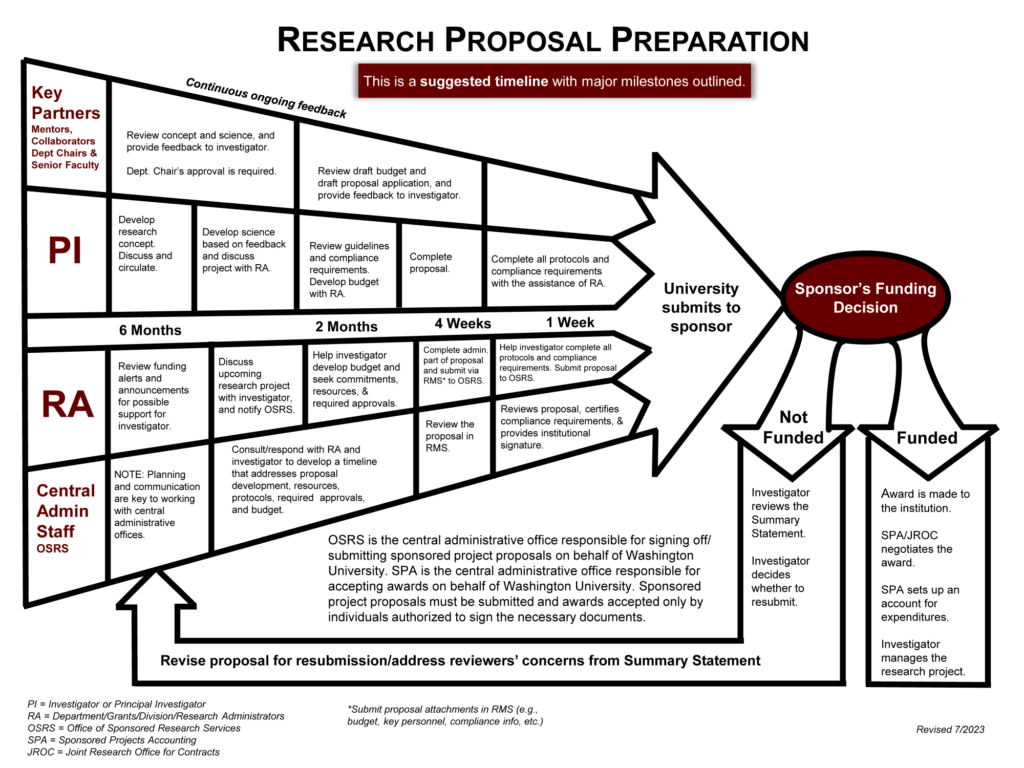
Proposal Timeline
The process to submit a proposal can take six months or longer, and requires the involvement of several people from departments and central offices.
Writing Resources
Grant Writing Resources: The OVCR shares a listing of several grant writing resources at the university and beyond.
ICTS – WUSTL Grant’s Library: This is a centralized resource for grant writers with access to samples of award training, career, and research grant applications (NIH, private, and institutional), as well as general stock language and proposal templates and tools.
Other Proposal Resources
Broader Impacts Partners and Resources: These resources help faculty define and develop their broader impacts as well as potential university and local partners.
Diversity, Equity, & Inclusion: This webpage provides a primer for the research community on activities and planning that build diversity, equity, and inclusion (DEI). The RDO encourages all researchers to utilize this primer, to adopt practices that connect to your research and build DEI. Further, researchers applying to funding opportunities that require DEI plans can utilize this primer to strengthen their application and their impact toward DEI.
Institutional Data: This list provides frequently requested institutional data for WashU.
Financial Disclosures: Disclosure is required for all External Professional Activities that appear to be related to a Covered Individual’s professional, academic, or scientific expertise or Institutional Responsibilities regardless of remuneration, unless otherwise exempted below. Covered entities include: all foreign and domestic governments, institutions and entities; public or non-publicly traded companies; non-profit organizations; university/academic organizations, and government and professional societies.
International Collaborators: Issues surrounding foreign influence and international activities in federally funded research has been an evolving topic, in which the US Government has shown increasing concern. With the heightened sensitivity around intellectual property and proper disclosures, it is imperative to report on international research and scholarly activities.
Transparency of Foreign Connections: The university has compiled a series of responses to the information requested from funding agencies, especially the DOE, related to foreign connections.
Controlled Unclassified Information (CUI): Security requirements for protection of CUI are required when the originating or immediate sponsor is the US Government, and the mechanism used to fund the work will be a contract or subcontract (rather than a grant or cooperative agreement).
Digital Personal Identifier (DPI): One of the NSPM-33 objectives is to strengthen disclosure requirements and processes. While it is not yet a requirement, it is recommended that individual researchers on any federal research grant register with a service that provides them with a digital persistent identifier. Currently ORCID is the tool accepted for DPIs by federal agencies. The Becker Library ORCID guide shares tutorials, videos, and other information on DPIs.
Biosketch: A collection of information on a researcher’s education, employment, research activities, publications, and more. A biosketch can be created with the SciENcv tool, and information can be made public to share with others. The biosketch may also include information on Other Support/Current & Pending Support. Sponsors may request information on other active and pending support to ensure there is no scientific, budgetary, or commitment overlap.
SciENcv: This tool allows researchers to track and share their biosketch and current and pending support. Biosketch samples can be found in the WUSTL Grants Library>Standard Grant Language Templates in Box.
Tips & Reminders for Pre-Award: OSRS provides a wealth of tips on how to submit your application on time and avoid errors.
Correcting an Application: Sometimes after an application is submitted you may want to pull it back from the sponsor to make an edit or correction.
- NIH: Submit a Changed/Corrected Application
- Research.gov: Proposal Submission>Proposal FAQs>Editing a Submitted Proposal
4: Submit Project
Research Management System (RMS)
Every proposal with a budget requirement (including pre-proposals) must go through RMS and the approval process. Because the system calculates salary and fringe rates, it is recommended to start this process early.
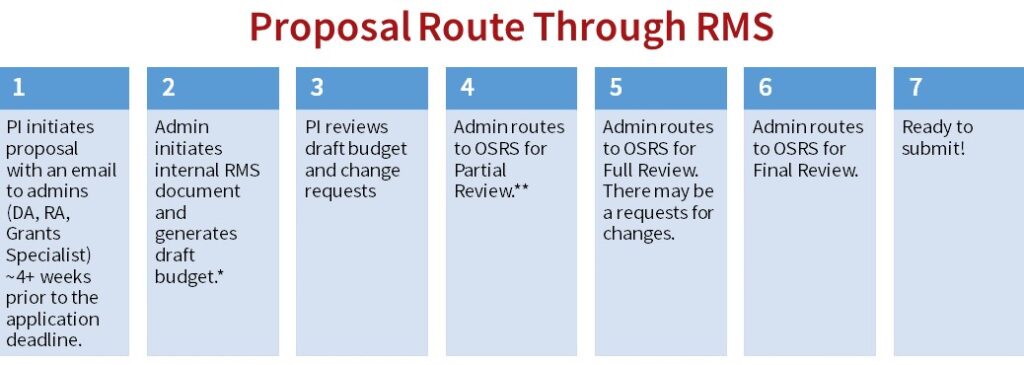
*Every proposal with a budget requirement (including pre-proposals) must go through RMS and the approval process. Because RMS calculates salary and fringe rates, do this early in the process.
**Once the budget meets the PI’s approval, admin routes to the central Office of Sponsored Research Services (OSRS)for Partial Review. OSRS may approve or request changes. Admin makes requested changes, in consultation with PI (if necessary). Once approved, admin generates Proposal Certification forms (PC forms) and sends to PI for signature, followed by the department chair.
OSRS recommended timeline for review:
- 10+ business days for Partial Review
- 5+ business days for Full Review
- 2+ business days for Final Review
| Institutional Signature Authority |
|---|
| PIs cannot sign agreements on behalf of WashU. OSRS has the institutional signature authority. |
5: Final Requirements Before Acceptance
Pre-Award Compliance
There are many policies and regulations setting the training requirements for researchers. The Trainings for Researchers page provides information on several of the required compliance trainings, as well as resources for staff to monitor completions. Failure to complete and submit all required training will delay the award acceptance.
Just-in-Time Procedures
If the NIH decides to fund your proposal, they must have certain information before making the award. This may include current other support, IRB approvals, IACUC approvals, human subjects education, SBIR/STTR funding agreements, and more. There are several important steps for both the PI and OSRS administrators to follow during the JIT process.